- 當(dāng)前位置:首頁(yè) >代理 > 美國(guó)SiELC > SHARC
- SHARC
SHARC? HPLC columns
SHARC? HPLC columns are the latest innovation from SIELC Technologies, the inventors of Primesep®. SHARC? columns are the first commercially available columns with separation based primarily on hydrogen bonding. SHARC? stands for Specific Hydrogen-bond Adsorption Resolution Chromatography.
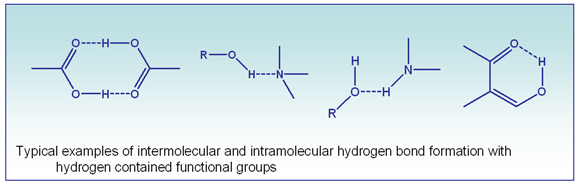
Hydrogen bonding is an interaction between hydrogen atom bound to electronegative atoms in a molecule, such as oxygen, nitrogen, or fluorine. This is typically a weak interaction, especially when separation is performed in aqueous solutions. Liquid chromatography techniques evolved as a tool for separation of different molecules based on their physico-chemical properties. Most common techniques of the separation are:
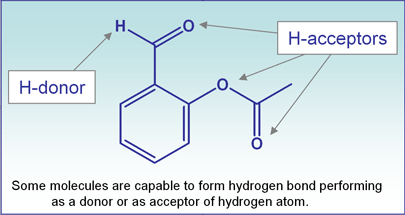
Columns and stationary phases based on these techniques never perform purely with one type of interaction. Hydrogen bonding is omnipresent in every one of these techniques with minor contribution to retention and selectivity. However, in some cases, especially in normal phase chromatography, the contribution of hydrogen bonding can be significant. SHARC? 1 is the first column specifically designed to perform a separation based entirely on the interaction of the molecules capable providing hydrogen atom (donor) or attract hydrogen atom (acceptor) to the stationary phase with special properties.
Technology
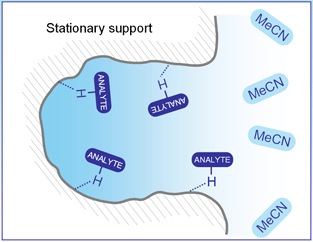
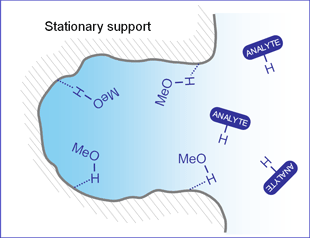
SHARC? 1 column operation conditions are unique. A mixture of acetonitrile (MeCN), a weak solvent, and methanol (MeOH), a strong solvent, are used as the mobile phase. Pure MeCN has very insignificant amount of hydrogen bonding with the SHARC stationary phase, while MeOH interacts strongly with SHARC? stationary phase, which reduces the retention of analytes based on its capacity to the hydrogen bond. By changing the ratio of MeCN/MeOH, the optimum retention profile can be obtained for many types of molecules with high selectivity, peak shape, efficiency, and speed.
|
|
Surface interaction within the column with hydrogen, bonding capable analytes with acetonitrile as the mobile phase. |
Surface interaction within the column with hydrogen, bonding capable analytes with methanol as the mobile phase. |
Hydrogen bonding energy is usually in range of 30 kJ/mol or less and depends strongly on the nature of the functional groups and their orientation within the molecule. This energy difference is the basis for selectivity of the interaction between the stationary phase and the molecule of different structure and chemical characteristics.A given molecule can retain the stationary phase with more than one hydrogen bond, while also performing as a donor or acceptor of a hydrogen atom.Presence of a polarized hydrogen atom is not always enough to observe retention by hydrogen bond mechanism. Sometimes a compound can form intra-molecular interactions, as opposed to inter-molecular hydrogen bonding, and does not participate in stationary phase interaction.SHARC? 1 column is a hydrogen acceptor type stationary phase showing higher retention characteristics toward molecules with higher number of polar X-H bonds such as alcohols, amines, acids, amides, phenols etc.
.
|

|
Advantages
SPEED
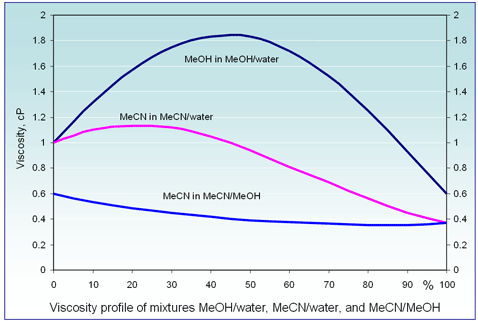
– MeCN/MeOH mixture has 2-3 times lower viscosity than H20/MeOH or H20/MeCN mixture (fig. 5). As result, smaller particles for column packing can be used without increasing the working pressure. UPLC-like conditions can be easily obtained on regular HPLC instrument with 2-3 times higher velocity with a shorter column with small particles. This can increase the speed of analysis up to 5 times. Using UPLC (RRLC, UHPLC) equipment allows to increase the speed of the analysis by another factor of 5 or 6.
SOLUBILITY
– MeOH is one of the most universal solvents for organic compounds. The combination of MeOH with MeCN allows to dissolve almost any molecules with high or low polarity. Hydrophobic molecules such as surfactants, lipids, and oil soluble vitamins, are soluble in this solvent combination. Very polar molecules such as sugars, di-ols, salts of amino compounds, or carboxylic acids also dissolve in this solvent system.
SELECTIVITY
– Since hydrogen bonds formation is very specific in the interaction energy and strongly depends on molecule geometry and a number of functional groups and the position of the functional groups, the selectivity of resolution of molecule of similar nature such as isomers, related impurities, product of oxidation or reduction can be achieved with SHARC? separation very efficiently.
PREPARATIVE SEPARATION
– MeCN/MeOH mixture has a low boiling point and is much easier to evaporate than water. As result, this solvent system is much friendlier for preparative type chromatography. The additional benefit of low viscosity allows SHARC? to perform prep separation with higher throughput. This mobile system is MS friendly and allows implementation of MS-driven sample collection. In most cases, an isocratic method can be used due to high selectivity of the column which allows to recycle most of the mobile phase minimizing solvent consumption.
WIDE RANGE OF COMPOUNDS
– Wide range of molecules can be analyzed with SHARC? technique. Practically any molecule with a functional group which contains oxygen and nitrogen can be retained and separated from similar compounds in this technology. Hydrocarbons is one class of compounds that will be retained poorly in this system, but they are typically well separated in reverse phase HPLC or GC.
Method Development
Hydrogen-bonding interaction offers unique selectivity based on number of “interaction points” available for hydrogen bonding. One of the useful characteristics to determine retention patterns in hydrogen-bonding mode is the molecular polar surface area (PSA). This calculated parameter is usually used for prediction of drug transport properties, but we successfully applied it to hydrogen-bonding interactions. Polar surface area is defined as a sum of surfaces of polar atoms (usually oxygens, nitrogens and attached hydrogens) in a molecule. Since those polar atoms can participate in hydrogen-bonding interaction the estimation of elution order can often be made based on PSA. While PSA is a good indicator of elution time, it must be noted that the polar surface area does not account for the accessibility of hydrogen-interaction sites. Not every polar surface participates in inter-molecular hydrogen interactions with the stationary phase.
Proximity of “interaction points” to each other within one molecule also needs to be considered since molecules can form an intra-molecular hydrogen-bonding, which competes with inter-molecular interaction between an analyte and stationary phase. This reduces retention time in hydrogen-bonding mode. Such structural factors provides unique selectivity among similarly structural (isomers, homologs, degradation products, precursors) molecules.
Since SHARC? 1 column is a mixed-mode column, pKa is another useful parameter in method development for these columns. SHARC? columns operate in non-aqueous mobile phase, but some effect of charge interaction of the stationary phase and ionizable molecules still exists and contributes to the retention profile.